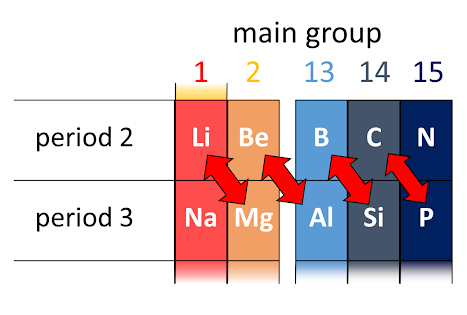
It can be seen from periodic table that some diagonally situated elements have same properties in the second and third period of the periodic table and it may be because of size_charge phenomenon.
An example of this relationship is given below;
Lithium_Magnesium:
There is a series of lithium alkyls and lithium aryls whish are useful in organic chemistry in much the same way as magnesium Grignard reagent.
The solubilities of several lithium compounds more nearly resemble those of the corresponding magnesium salts than of other alkali metal salts.
The Diagonal Relationship