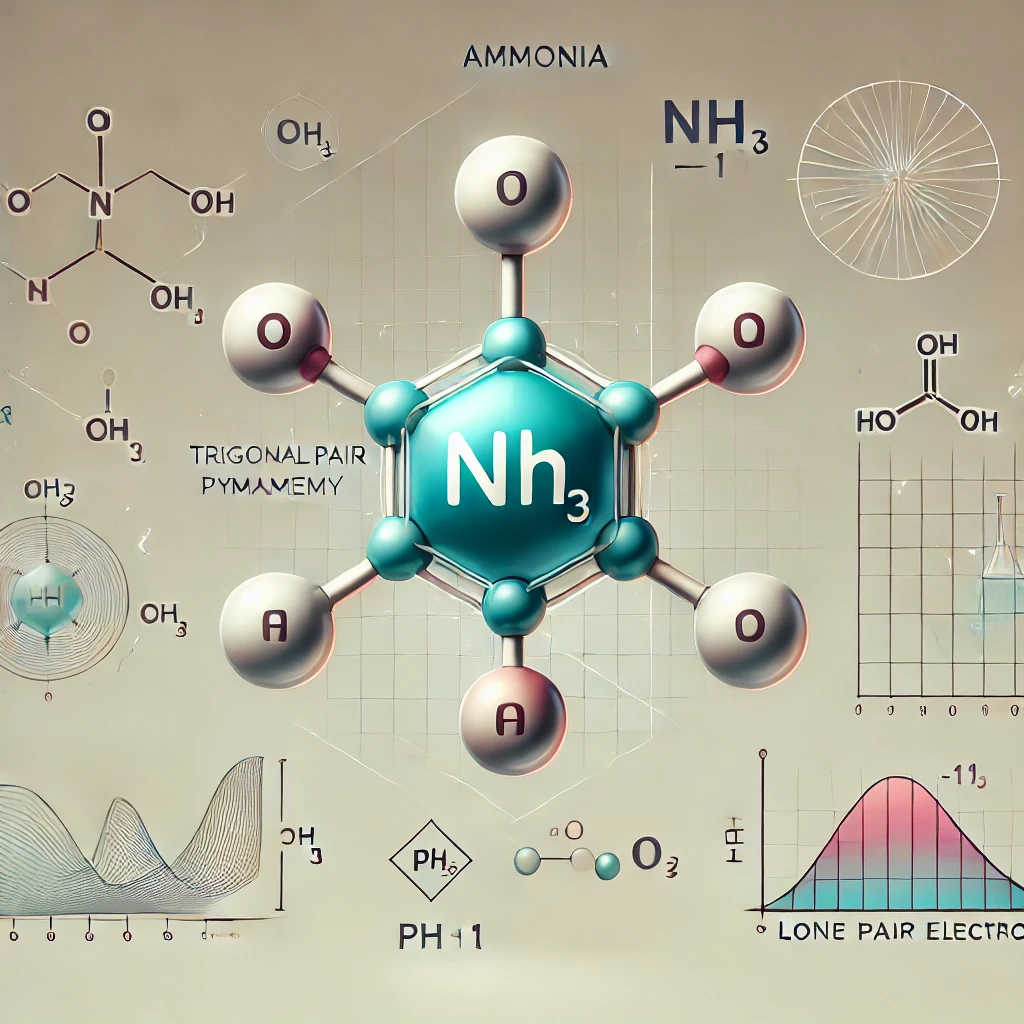
Is Ammonia trigonal Pyramidal or Planar?
Ammonia is trigonal pyramidal and not trigonal planar .
Reason:
This is because ammonia has a lone pair of electron . In the structure of ammonia three hydrogen bonds are shown below and lone pair is shown above the nitrogen atom. As we know that , lone pair occupies more space than the bond pair so
The upper lone pair repels bond pair and due to this repulsion bond pair bends .
That’s the reason why ammonia has trigonal pyramidal shape.
Ammonia (NH3)
It is the powerful gas which is composed of nitrogen and hydrogen. It is colorless. It’s the only stable compound of those elements and is a starting material for the assembly of the many commercially important nitrogen compounds.
Uses of ammonia
Ammonia is mostly used as a fertilizer within the us. It’s usually applied on to the soil from tanks containing the liquefied gas. The ammonia also can be within the sort of ammonium salts, like nitrate , NH4NO3, ammonium sulfate, (NH4)2SO4, and various ammonium phosphates. Urea, (H2N)2C=O, is that the most ordinarily used source of nitrogen for fertilizer worldwide. It is utilized in the manufacture of economic explosives (e.g., trinitrotoluene [TNT], nitroglycerin, and nitrocellulose).
In the textile industry, ammonia is employed within the manufacture of synthetic fibres, like nylon and rayon. additionally. It’s employed within the dyeing and scouring of cotton, wool, and silk. Ammonia is a catalyst within the production of some synthetic resins. More important, it neutralizes acidic by-products of petroleum refining, and within the rubber industry it prevents the coagulation of raw latex during transportation from plantation to factory. Ammonia also finds application in both the ammonia-soda process (also called the Solvay process), a widely used method for producing sodium carbonate , and therefore the Ostwald process, a way for converting ammonia into aqua fortis .
Ammonia is employed in various metallurgical processes, including the nitriding of alloy sheets to harden their surfaces. Because ammonia are often decomposed easily to yield hydrogen. It’s a convenient portable source of atomic hydrogen for welding. Additionally , ammonia can absorb substantial amounts of warmth from its surroundings (i.e., one gram of ammonia absorbs 327 calories of heat), which makes it useful as a coolant in refrigeration and air-conditioning equipment.
Preparation of ammonia
In 1774 By the English Physical Scientist Priestley Pure ammonia was first prepared, and in 1785 the French chemist Claude-Louis Berthollet decided its exact composition. Ammonia is consistently among the highest five chemicals produced within the us . The chief commercial method of manufacturing ammonia is by the Haber process , which involves the direct reaction of elemental hydrogen and elemental nitrogen.
N2 + 3H2 → 2NH3
This reaction requires the utilization of a catalyst, high (100–1,000 atmospheres), and elevated temperature (400–550 °C [750–1020 °F]). Actually, the equilibrium between the weather and ammonia favours the formation of ammonia at coldness , but heat is required to realize a satisfactory rate of ammonia formation. Several different catalysts are often used. Normally the catalyst is iron containing iron oxide. However, both periclase on alumina that has been activated by alkaline metal oxides and ruthenium on carbon are employed as catalysts. within the laboratory, ammonia is best synthesized by the hydrolysis of a metal nitride.
Mg3N2 + 6H2O → 2NH3 + 3Mg(OH)2
Physical properties of ammonia
Ammonia may be a colorless gas with a pointy , penetrating odour. Its boiling point is −33.35 °C (−28.03 °F), and its melting point is −77.7 °C (−107.8 °F). it’s a high heat of vaporization (23.3 kilojoules per mole at its boiling point) and may be handled as a liquid within the rmally insulated containers in the laboratory. (The heat of vaporization of a substance is that the number of kilojoules needed to vaporize one mole of the substance with no change in temperature.)
The ammonia molecule features a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom. It’s a polar molecule and is very associated due to strong intermolecular hydrogen bonding. The dielectric constant of ammonia (22 at −34 °C [−29 °F]) is less than that of water (81 at 25 °C [77 °F]), so it’s a far better solvent for organic materials. However, it’s still high enough to permit ammonia to act as a moderately good ionizing solvent. Ammonia also self-ionizes, although less so than does water.
2NH3 ⇌ NH4+ + NH2−
Chemical reactivity of ammonia
The combustion of ammonia proceeds with difficulty but yields nitrogen gas and water.
4NH3 + 3O2 + heat → 2N2 + 6H2O
However, with the utilization of a catalyst and under the right conditions of temperature, ammonia reacts with oxygen to supply gas , NO, which is oxidized to dioxide , NO2, and is employed within the industrial synthesis of aqua fortis .
Ammonia readily dissolves in water with the liberation of warmth .
NH3 + H2O ⇌ NH4+ + OH−
These aqueous solutions of ammonia are basic and are sometimes called solutions of ammonia water (NH4OH). The equilibrium, however, is such a 1.0-molar solution of NH3 provides only 4.2 millimoles of hydroxyl ion . The hydrates NH3 · H2O, 2NH3 · H2O, and NH3 · 2H2O exist and are shown to contains ammonia and water molecules linked by intermolecular hydrogen bonds.
Physical measurements, including electrical-conductivity studies, provide evidence that this blue colour and electrical current are thanks to the solvated electron.
metal (dispersed) ⇌ metal(NH3)x ⇌ M+(NH3)x + e−(NH3)y
Because the concentration of dissolved metal increases, the answer becomes a deeper blue in colour and eventually changes to a copper-coloured solution with a metallic lustre. The electrical conductivity decreases, and there’s evidence that the solvated electrons associate to make electron pairs.
2e−(NH3)y ⇌ e2(NH3)y
Usually the salt of ammonia also readily dissolve in the liquid of ammonia
Derivatives of ammonia
Two of the more important derivatives of ammonia are hydrazine and hydroxylamine.
Hydrazine
Hydrazine, N2H4, may be a molecule during which one atom in NH3 is replaced by an ―NH2 group. The pure compound may be a colourless liquid that fumes with a small odour almost like that of ammonia. The physical properties of it are as : It’s freezing point of two °C (35.6 °F), and the boiling point of 113.5 °C (236.3 °F), and high dielectric constant at (51.7 at 25 °C [77 °F]), and a density of 1 gram per cubic cm. like water and ammonia, the principal intermolecular force is hydrogen bonding.
Hydrazine is best prepared by the Raschig process, which involves the reaction of an aqueous alkaline ammonia solution with hypochlorite (NaOCl).
2NH3 + NaOCl → N2H4 + NaCl + H2O
Ammonia reacts rapidly and quantitatively with the hypochlorite ion, OCl−, to supply chloramine, NH2Cl, which reacts further with more ammonia and base to supply hydrazine.
NH3 + OCl− → NH2Cl + OH− NH2Cl + NH3 + NaOH → N2H4 + NaCl + H2O
In this process there’s a detrimental reaction that happens between hydrazine and chloramine which appears to be catalyzed by heavy metal ions like Cu2+.
N2H4 + 2NH2Cl → 2NH4Cl + N2
When hydrazine is added to water, two different hydrazinium salts are obtained. N2H5+ salts are often isolated, but N2H62+ salts are normally extensively hydrolyzed.
N2H4 + H2O ⇌ N2H5+ + OH− N2H5+ + H2O ⇌ N2H62+ + OH−
Hydrazine burns in oxygen to supply nitrogen gas and water, with the liberation of a considerable amount of energy within the sort of heat.
N2H4 + O2 → N2 + 2H2O + heat
As a result, the main noncommercial use of this compound (and its methyl derivatives) is as a rocket propellant . Hydrazine and its derivatives are used as fuels in guided missiles, spacecraft (including the space shuttles), and space launchers. For instance , the Apollo program’s lunar excursion module was decelerated for landing, and launched from the Moon, by the oxidation of a 1:1 mixture of methyl hydrazine, H3CNHNH2, and 1,1-dimethylhydrazine, (H3C)2NNH2, with liquid dinitrogen tetroxide, N2O4.
Three plenty of the methyl hydrazine mixture were required for the landing on the Moon, and about one ton was required for the launch from the lunar surface. The main commercial uses of hydrazine are as a blowing agent (to make holes in foam rubber), as a reducer , within the synthesis of agricultural and medicinal chemicals, as algicides, fungicides, and insecticides, and as plant growth regulators.
Hydroxylamine
Hydroxylamine, NH2OH, could also be thought of as being derived from ammonia by replacement of a atom with a hydroxyl (―OH). The pure compound may be a colourless solid that’s hygroscopic (rapidly absorbs water) and thermally unstable. It must be stored at 0 °C (32 °F) in order that it’ll not decompose. It melts at 33 °C (91.4 °F), features a density of 1.2 grams per cubic cm at 33 °C, and features a high dielectric constant (ε = 78).
Aqueous solutions of hydroxylamine aren’t as strongly basic as either ammonia or hydrazine. Hydroxylamine are often prepared by variety of reactions. A laboratory synthesis involves the reduction of aqueous potassium nitrite, KNO2, or acid , HNO2, with the hydrogen sulfite ion, HSO3−. Generally , hydroxylamine is stored and used as an solution or as a salt (for example, NH3OH+NO3−). It’s often utilized in the preparation of oximes.
VSEPR NH3: Valence Shell Electron Pair Repulsion Ammonia
Ammonia has 4 regions of electron density round the central nitrogen atom (3 bonds and one lone pair). These are arranged during a tetrahedral shape. The resulting molecular shape is trigonal pyramidal with H-N-H angles of 106.7°.
Why is ammonia NH3 pyramidal and not trigonal planar (flat)?
It is trigonal pyramidal due to the lone pair of electrons related to the central nitrogen atom.
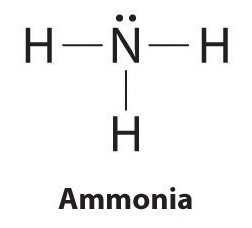
Explanation:
The Lewis structure for ammonia seems like this…
You can see the lone pair (nonbonding) of electrons directly above the nitrogen.
The nonbonding pair of electrons pushes faraway from the bonding pairs producing a trigonal pyramidal shape. If the central atom with no lone pair is bonded to 3 other atoms the molecule will have a trigonal planar shape.
The pH Level of Ammonia
Ammonia may be a common liquid utilized in households and industry, easily identified by its distinctive smell. The quality pH of ammonia explains many of the properties of the chemical.
Ammonia may be a weak base with a typical pH level of about 11.
pH of Ammonia
One molecule of ammonia consists of 1 negatively-charged nitrogen ion and three positively-charged hydrogen ions, giving ammonia a formula of NH3. The pH of ordinary ammonia is about 11.
Features of Ammonia
Ammonia may be a base, which suggests it reacts in water to make a positively-charged ammonium (NH4+) ion and a negatively-charged hydroxide (OH-). As a base, ammonia features a bitter taste (although you ought to never taste it), a soapy feel and therefore the ability to neutralize acids. Ammonia may be a weak base, so it only exhibits the common corrosive effects of the many bases when in high concentration.
Formation of Ammonia
Ammonia occurs naturally and may even be manufactured. Natural ammonia, which exists in trace quantities within the atmosphere, typically comes from the decomposition of organic matter. Most ammonia, however, is made via chemical processes that bond together the nitrogen and hydrogen ions.
Safety Warnings
Ammonia inhalation, which is typically avoidable thanks to the strong smell, can cause serious respiratory distress. Highly concentrated ammonia can burn the skin or eyes. Treatment involves flushing the affected area with water. If you ingest ammonia, don’t induce vomiting but seek medical help immediately. If you employ ammonia as a household cleaner, be especially careful to never mix the substance with bleach. A deadly gas called chloramine is that the results of such a mix .
Benefits of Ammonia
Ammonia mixes with water and is employed in many household cleaners. Most of those cleaners contain between 5 percent and 10 percent ammonia by volume. Commercial cleaners also use ammonia, but the concentrations are much greater, being as high as 25 percent to 30 percent ammonia. Ammonia is additionally widely used as an ingredient in fertilizers, where it provides nitrogen to the soil. High concentration ammonia is additionally wont to etch metals and supply commercial refrigeration.
Why does my sweat smell like ammonia?
Not all kinds of sweat have an odor. However, sometimes sweat can smell like ammonia. this will flow from to a high protein diet, exercise, or health conditions like renal disorder .
The purpose of sweat is to assist the body calm down. This will make the skin feel cool and reduce a person’s blood heat .
What is sweat?
Sweat can act as a barrier against bacteria on the skin, and it also can moisturize the skin.
Sweat contains water and common salt , also as small amounts of:
- potassium
- calcium
- ammonia
- urea
- lactate
- ethanol
A person may sometimes be ready to smell the ammonia content in their sweat.
Sweat glands everywhere the body release sweat. There are three sorts of sweat glands, which are called the eccrine, apocrine, and apoeccrine glands.
Eccrine glands are the foremost abundant across the body. this sort of sudoriferous gland produces the foremost sweat, but it doesn’t have a smell.
Apocrine glands are found in specific areas of the body, like the breasts, face, scalp, perineum, and therefore the underarms.
The apocrine glands produce sweat that comes through hair follicles instead of through the pores on the skin. Sweat from apocrine glands can have an odor. It contains high levels of lipids, also as sugars and ammonia.
Apoeccrine glands are only located within the underarms. Apoeccrine glands release sweat onto the skin’s surface within the sort of salt water.
Sweat from apoeccrine glands isn’t thought to assist regulate a person’s temperature because it cannot evaporate easily from the underarms.
What is ammonia?
Ammonia (NH3) may be a colorless gas that’s a compound of nitrogen and hydrogen. it’s a robust odor that smells like urine or sweat.
Ammonia occurs naturally in water, soil, and therefore the air, and is additionally found naturally in plants, animals, and therefore the physical body .
Causes of odor in sweat
There are many possible causes of sweat smelling like ammonia. These include:
Diet
The diet of person can make their sweat smell like ammonia.
A person who eats a diet high in protein but low in carbohydrates may produce ammonia that the body then releases in sweat.
The body usually uses carbohydrates for energy by converting them into glucose because carbohydrates are the fastest energy supply. However, if there aren’t enough carbohydrates, the body will use protein for energy.
The body then releases this ammonia through urine and sweat, which can produce an odor.
Dehydration also can make the sweat smell like ammonia. this is often because the body needs water to urge obviate ammonia through sweat.
If there’s not enough water to dilute the ammonia because it is released by the body, the smell of ammonia could also be stronger.
Exercise
Some research suggests exercise affects ammonia levels in sweat. A 2007 study found that ammonia levels in sweat increased as an individual exercised more intensely.
This suggests an individual may find their sweat smells most strongly of ammonia during or simply after exercise.
Health conditions
Certain health conditions can cause a person’s body odour to vary . They include:
Hyperhidrosis
Hyperhidrosis causes excessive sweating from the eccrine glands. A 2016 study estimated 15.3 million people within the us accept hyperhidrosis.
There are two sorts of hyperhidrosis:
- Primary focal hyperhidrosis: this sort of hyperhidrosis can affect the underarms, hands, feet, and forehead. Primary focal hyperhidrosis isn’t thanks to an underlying health condition or a side effect from medication.
- Secondary hyperhidrosis: this sort of hyperhidrosis is thanks to an underlying health condition or a side effect of medication.
If an individual sweats tons , sweat may build abreast of their skin and interact with bacteria. this will cause an odor to develop, which can smell like ammonia.
Kidney disease
Kidney disease can cause a person’s body odour to vary .
The kidneys are liable for removing urea from the body. However, if the kidneys aren’t working properly, urea may enter into the bloodstream instead. this is often called uremia, and may be a serious symptom of renal failure .
If an individual has high levels of urea in their body, the body may release urea through sweat and cause an odor.
According to the National Kidney Foundation, the most causes of renal disorder are diabetes and high vital sign , which accounts for up to 66% of cases.
Diabetes
A complication of diabetes may be a condition called diabetic ketoacidosis (DKA).
DKA develops when the body burns fat for energy if there’s not enough insulin to use glucose within the cells.
A kind of ketone called acetone can make the breath smell fruity or change a person’s body odour .
If the extent of ketones becomes too high, the blood can become too acidic, which may be life-threatening.
According to the American Diabetes Association, DKA is rare in people with type 2 diabetes, but it can still develop in anyone.
DKA produces symptoms including:
- frequent urination
- thirst
- very xerostomia
- high blood glucose levels
- high ketone levels in urine
- vomiting
Trichomycosis
Trichomycosis may be a bacterial infection that affects the hair within the underarms. consistent with a 2013 study, it can affect bush in rare cases.
The bacteria Corynebacterium causes trichomycosis and produces yellow, black, or red nodules that stick with the hairs.
Odor was a symbol of trichomycosis in 35.7% of cases, consistent with the study of 2013.
Trimethylaminuria
Trimethylaminuria may be a rare condition. Increased levels of the compound trimethylamine (TMA) within the body cause trimethylaminuria. TMA features a fish-like smell.
Bacteria within the gut produces TMA. consistent with the National Institutes of Health, an individual with trimethylaminuria may have inherited a faulty gene meaning the body cannot break down TMA properly.
Treatment can include taking activated carbon supplements to assist remove excess TMA from the body. B12 supplements, antibiotics, and probiotics can also help.
Meat consumption
Dietary choices may affect body odour , including whether people eat meat or not.
An older study from 2006 found that participants on a meat diet had body odour which others perceived as less attractive than the body odour of participants on a non-meat diet.
Dairy consumption
In rare cases, eating eggs and can cause a person’s sweat to smell different. this is often because dairy products produce trimethylamine when the body digests them, which features a strong, fishy smell.
A 2015 study found that trimethylamine-N-oxide (TMAO) levels increased after the participants ate dairy products. Trimethylamine produces TMAO.
Although trimethylamine-N-oxide is odorless, if an individual cannot digest trimethylamine properly, the body may release it through sweat and cause body odour .
Spices and seasonings
Spices like cumin or curry can change a person’s sweat odor. this is often because they will create sulfur-like compounds when digested, which may react with sweat and cause odor.
Onions, herbs, and garlic also can have an equivalent effect.
Stress
A person may sweat thanks to stress. The International Hyperhidrosis Society states that the apocrine glands are liable for producing stress-related sweat.
When this sort of sweat sits on the skin and mixes with bacteria, it can create a smell.
Hormones
A person’s hormones may change during puberty, menstruation, pregnancy, and menopause.
Decreasing estrogen levels during menopause may cause sweating, which may increase odor if it mixes with bacteria on the skin.
Prevention
People can take several steps to stop sweat smelling like ammonia. These include:
Changing the diet
To prevent the body from using protein as energy after exercise, which can cause their sweat to smell like ammonia, an individual can increase the quantity of carbohydrates in their diet.
Whole grains, vegetables, fruits, and beans are healthy sources of carbohydrates.
Additionally, if an individual notices that certain foods, like fish or dairy, make their sweat smell like ammonia, they will reduce their intake of these foods or remove them from their diet.
Drinking more water
Increased water intake can dilute sweat and make its odor less noticeable.
Deodorants and anti-perspirants
A person can use deodorants to hide up the ammonia smell from sweat.
They can also try antiperspirants, which block the sweat glands and stop sweating from happening.
Changing clothes
A person can try changing their clothes more frequently to stay their skin dry and freed from bacteria which will react with sweat and cause odor.
Washing
Antibacterial soap can fight bacteria on the skin. this may keep the skin clean and fight any odor from bacteria mixing with sweat.
Antifungal creams or powders also can help an individual reduce their risk of fungal infections.
Reducing stress
If an individual notices they sweat more with stress, they will try evaluating the sources of stress in their life.
If they’re ready to reduce their stress levels, they’ll sweat less and reduce any odors that were occurring due to their stress.
When to ascertain a doctor
If their urine features a strong ammonia smell, this might be a symbol of diabetes. If an individual notices blood in their urine, or that their urine is foamy, this might be a symbol of renal disorder .
Fungal infections may cause rashes, sores, or blisters. an individual should contact a doctor if they think they’ll have a fungal skin infection.
Treatment
To help control body odour , the International Hyperhidrosis Society recommends:
- keeping the skin dry
- washing with antibacterial soap
- reducing sweating by using antiperspirants
- masking odors with deodorants
A person also can contact a doctor to debate the subsequent medical treatments:
Microwave thermolysis
Microwave thermolysis may be a treatment that uses microwave energy to prevent the sweat glands from working properly.
A 2013 study found that microwave thermolysis was an efficient treatment for sweat that produced odors.
Botox
The U.S. Food and Drug Administration (FDA) has approved the utilization of onabotulinumtoxinA, or Botox, to treat excessive sweating.
Botox are often an efficient treatment for hyperhidrosis because it blocks the chemical that signals for the sweat glands to supply sweat.
According to the International Hyperhidrosis Society, Botox can cause an 82-87% reduction in underarm sweating.
Botox injections are often effective in stopping underarm sweating for 4-12 months, and possibly up to 14 months.
Prescription antiperspirants
The International Hyperhidrosis Society recommends antiperspirants to assist control excessive sweating and body odour .
Prescription antiperspirants may contain higher amounts of active ingredients than over-the-counter antiperspirants.
These active ingredients can include metallic salts like aluminium chloride hexahydrate.
Prescription antiperspirants are best when people apply them both within the dark and in the morning.
Antibiotics
A person can use antibiotics to treat underlying conditions that cause sweat to smell like ammonia, like trichomycosis.
Summary
Many things can influence the smell of a person’s sweat. Diet, exercise, and bacterial infections may all alter body odour .
A person living with a health condition like diabetes or renal disorder can also have sweat that smells like ammonia.
A person can try antiperspirants to scale back the quantity they sweat, and deodorants to hide up any odors. A doctor can treat any underlying health conditions to assist reduce the ammonia smell in sweat.
Read more about: What is an acid?
Read more about: What is a base?
Read more about: What is an alkali?
Read more about: Difference between alkali and base