Electronegativity:
Electronegativity is the tedency of an atom to draw electrons toward itself .
Electropositivity is it’s opposite,it is the tendency of an atom to donate electrons.
Variation of electronegativity in periodic table:
☆In going down the group , as the atomic number increases the size of tha atom also increasing because of increase in the number of shells . In this case nucleus can’t attract electrons strongly and hence the electronegativity decreases while going down the group.
☆In moving from left to right in a period the sizes of the atom decreases and nucleus can attract electrons strongly so the electronegativity increases while moving in a periid from left to right.
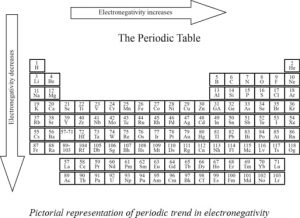
Electronegativity and it's variation in periodic table

Thank uhh?