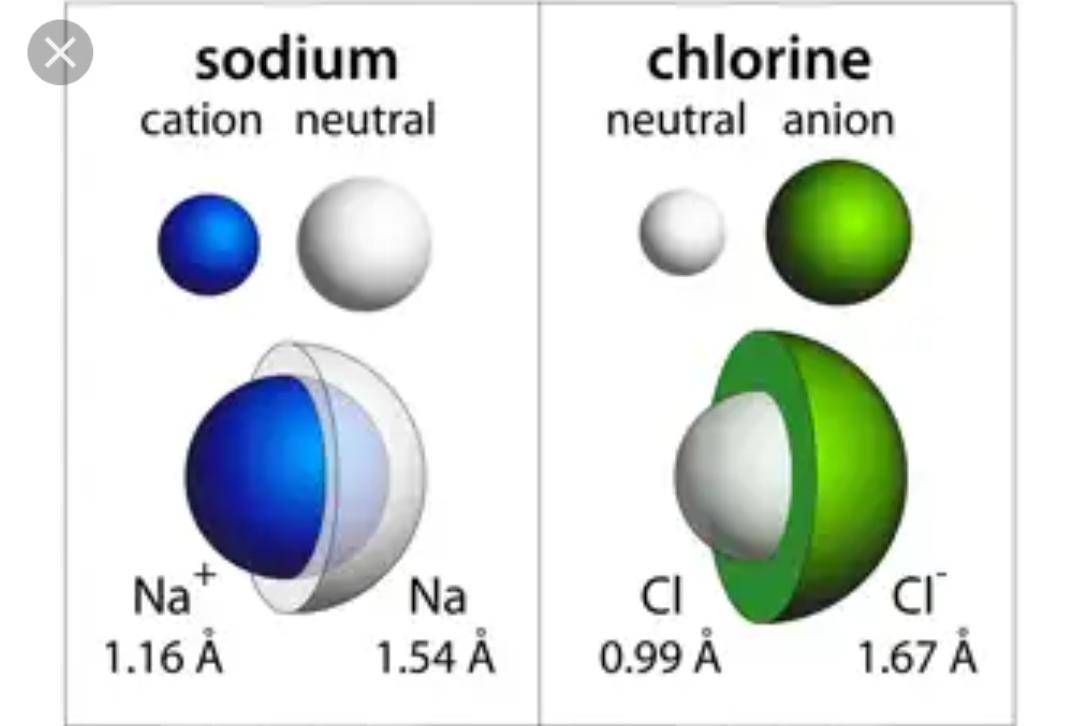
Size of Cation:
Size of the cation is larger than their parent atom.
Reason:
” Any specie containing positive charge due to the loss of one or none electron is called cation”.
As an atom releases one or more electron the electron_electron repulsion decreases and hence the remaining electrons are attracted and held by the nucleus strongly. That’s why the size of cation is smaller than it’s parent atom.
Example:
When sodium matel loses an electron it is converted into cation Na+. The size of Na+ is less than Na metal.
The ionic radius of sodium metal is 186pm
While the radius of it’s cation is 95pm.
Size of anion:
Size of anion is greater than it’s parent atom.
Reason:
“Any specie containing negative charge by the acceptance of one or more electron is called anion”.
When an atom accepts one or more electron,the number of electron around the nucleus increases and electron_electron repulsions also increases which results in the expansion of charge density and the size of atom also increases .This is the reason why Size of an anoin is greater than its parent atom.
Example:
When chlorine accepts an electron it is converted into anion .The ionic radius of chlorine atom is 99pm while the ionic radius of it’s anion is 181pm.
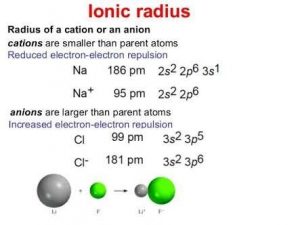
Comparison of Size of cation and anion with their parent atom