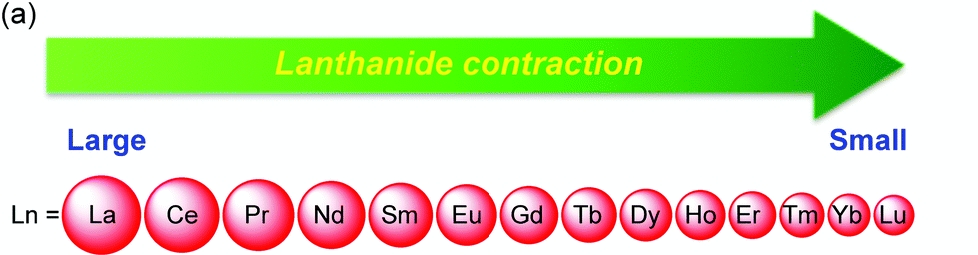
“Lanthanide contraction is the steady decrease in the size of elements of lanthanide series with the increase in atomic number from Lanthanum (atomic no: 57 ) to Lutetium (Atomic no:71)”.
CAUSE of lanthanide contraction:
》lanthanide contraction occurs due to the poor shielding of 4f electrons .As we move from Ce to Lu the addition of one extra electron take place that’s why 4f electron has a very diffused shape and a poor shielding ability.
》As we move from La to Lu the atomic number increaaes with the increase in the number of electron the attraction between the nucleus and the electron increases due to poor shielding of 4f electron and hence the size of atom decreases from La to Lu.
This decrease in size due to increase in atomic number can be explained by plotting a graph between atomic number on x_axis and ionic radius on y_axis
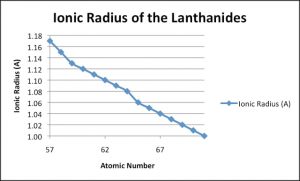
It can be seen from the graph that when atomic number increases the ionic radius decrease and the hence the size also decreases while moving toward Right.
Lanthanide contraction

Good fabulous???